Emergency Medicine Research: Anaphylaxis and Epinephrine
About Dr. Julie Brown
 I’m an emergency medicine attending physician and co-director of Emergency Medicine Research at Seattle Children's, and associate professor in the Department of Pediatrics at the University of Washington School of Medicine. My research interests include anaphylaxis, minor procedures, ear and nose foreign bodies, aerodigestive foreign bodies, and emergent management of respiratory diseases. Read my profile.
I’m an emergency medicine attending physician and co-director of Emergency Medicine Research at Seattle Children's, and associate professor in the Department of Pediatrics at the University of Washington School of Medicine. My research interests include anaphylaxis, minor procedures, ear and nose foreign bodies, aerodigestive foreign bodies, and emergent management of respiratory diseases. Read my profile.
- Send me an email.
Current Research
The focus of my research is anaphylaxis and the use of epinephrine auto-injectors. Below are some of the projects my team is working on.
Anaphylaxis Clinical Standard Work Outcomes
<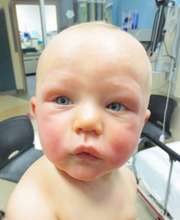 Infant experiencing anaphylaxis
Infant experiencing anaphylaxis
This study uses quality improvement methodology to look at quality of anaphylaxis care after improving and standardizing practice using a rigorous clinical standard work process. See poster.
- Status: Seeking publication. The abstract presented at the ACAAI meeting in Seattle last fall and will be presented at the 9th Annual Academic Pediatric Association Conference: Advancing Quality Improvement Science for Children’s Health, April 26, 2019 in Baltimore. The full manuscript has been submitted for publication.
- Funding needs: Manuscript submission costs, if needed
Poison Center Auto-Injector Injuries
This is a descriptive study of nearly 1,000 epinephrine auto-injector injuries reported to the Washington State Poison Control Center over 15 years. We reviewed the details of each call to learn the circumstances around each event, and better understand what prevention strategies might prevent future events.
- Status: The chart review is complete. Data is being analyzed and prepared for publication. An abstract was submitted and will be presented at Seattle Children’s Center for Clinical and Translational Research Science Day in May. An abstract may also be submitted to a national allergy meeting, funds permitting.
- Funding needs: Funds to support abstract and manuscript submission costs. Funds to cover travel to present at an allergy meeting.
A Novel Time-Based Clinical Score Guiding Epinephrine Treatment Decisions: the Anaphylaxis Score Assisting Providers (ASAP)
I developed a new clinical score to aid decision-making around the use of epinephrine, for patients presenting to care settings with allergic symptoms concerning for anaphylaxis. This study is the first validation study of this scoring tool.
- Status: Study complete, preparing for publication
- Funding needs: Manuscript submission costs, if needed.
Read the Seattle Children’s anaphylaxis pathway CSW developed through a rigorous clinical standard work process.
Pediatric Anaphylaxis Database
We are preparing a database of all visits to our pediatric emergency department and urgent cares.
- Status: Preparing for chart review
- Funding needs: Salary support for research assistant to perform chart review.
This will facilitate a number of studies:
- A study of the symptoms, signs and outcomes of infants <15 kg who receive epinephrine prior to arrival, most of which exceeds the recommended dose of 0.01 mg/kg.
- A study of persistent symptoms and signs and subsequent epinephrine use in patients whose initial epinephrine dose is delivered in the deltoid muscle of the arm rather than in the thigh
Epinephrine pharmacokinetics and pharmacodynamics before and during anaphylaxis
We are preparing to study epinephrine levels before and after food challenges, in high-risk patients receiving challenges in a hospital setting. All these patients have an IV placed on arrival, allowing for frequent blood draws. If epinephrine is given for anaphylaxis, epinephrine pharmacodynamics (blood levels vs symptoms) will be documented. This has never been done previously in anaphylaxis.
- Status: Preparing grant application
- Funding needs: This will need large grant support, such as NIH funding (applying for an R01). A small amount of funding will support grant preparation.
The Effects of Freezing on EpiPen® Epinephrine Auto-Injector Device Function and Integrity
To determine whether freezing and thawing of an EpiPen cracks or damages the glass epinephrine-containing vial or otherwise damages the device, we studied 104 pairs of expired post-consumer devices, 52 pairs of EpiPens and 52 pairs of EpiPen Jrs. Each pair had same lot number, expiration date, and consumer. One device in each pair was frozen at -25°C for 24 hours and then thawed, and the other was stored within the recommended storage temperature range. Both devices of the pair were then fired into meat and we used change in weight of the device and the meat to determine the amount of medication fired. We compared results for the frozen-thawed device with its paired non-frozen device. We also froze and thawed another 104 devices and opened them without firing, to inspect them for damage. Read more.
- Status: This study was presented at the ACAAI meeting in Seattle. The results are being prepared for publication.
- Funding needs: Likely none
Recent Podcast Features
Compassion For Children With Food Allergies
Pediatrician Dr. Wendy Sue Swanson highlights the importance of extending support, compassion and understanding to children and families with food allergies. Special guest Dr. Julie Brown, who works in Emergency Medicine at Seattle Children’s, provides her clinical expertise on anaphylaxis and anecdotal knowledge from parent support groups for children with life-threatening allergies.
Magnets, Batteries and Other Things Kids Swallow
Magnets, batteries and other small object can cause serious harm and even death. Pediatricians Drs. Wendy Sue Swanson and Julie Brown share what you need to know about what happens when toddlers and teens swallow magnets or batteries.